Many solutions are up in the air – quite literally. We can use a number of airborne gases to make products that are important to humankind and save resources and energy while we’re at it. For instance, we have been able to recover oxygen and nitrogen from the atmosphere through air distillation for a century already. Hospitals use liquid nitrogen to freeze important substances and liquid oxygen to treat patients with breathing difficulties. Rarer gases are also in regular use.
“The gaseous molecules in the atmosphere are already in good use; but there is still a need for more selective separation processes that consume less energy and for more efficient recycling,” says Nima Rezaei, assistant professor of gas separation at LUT University’s separation science department.
The atmosphere contains tens of gases
- Our atmosphere consists mostly of nitrogen (78%) and oxygen (21%).
- There are also small amounts of argon in the air (0.9%).
- In addition, trace gases such as noble gases, carbon dioxide, methane, hydrogen, ammonia, and ozone can be detected.
- The water vapor content in the air varies with elevation and location: it’s at its lowest near the polar regions and at its highest near rain forests.
Source: LUT University and the European Environment Agency
LUT recently opened a new gas separation laboratory on its campus in Lappeenranta and uses it to study carbon capture, utilization and storage, power-to-x technology, hydrogen storage, and industrial gas separation. Rezaei also aims to use the new lab to study air pollution control. He’s interested in researching the high prevalence of asthma in Finland and how it might be related to indoor air pollution.
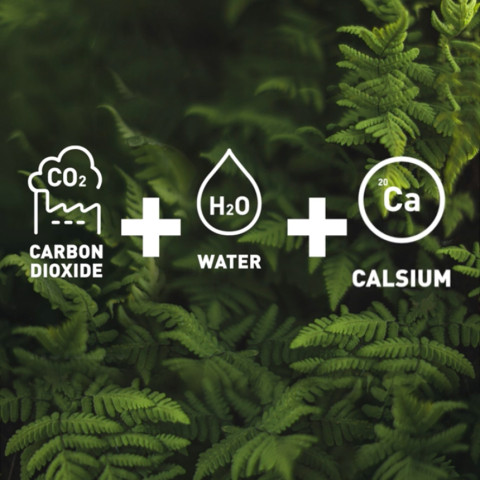
Turning carbon dioxide into industrial products
LUT’s strategy stresses that emissions may turn out to be an important industrial raw material down the line. Substances such as carbon dioxide could be used for the production of food, chemicals and fuels. LUT is exploring ways to capture carbon dioxide from the atmosphere and utilize it as efficiently as possible.
The research team of Tuomas Koiranen, professor of separation technology, has studied how the same process equipment could not only capture carbon dioxide but also produce calcium carbonate from it directly. Industry uses calcium carbonate widely to manufacture steel, glass, paper and paint and as a filler in toothpaste, pharmaceutical products and food.
Currently, calcium carbonate is produced from carbon dioxide that contains impure flue gases. This affects the quality of the end-product. Moreover, Koiranen points out that the resource and energy efficiency of the processes stand improving.
“The purity and further processing of the calcium carbonate are the greatest advantages of the process we are studying. LUT’s process yields ready-to-use micro-sized calcium carbonite crystals that need no further grinding. The crystals contain no impurities, which is especially important in the pharmaceutical industry,” explains Koiranen, who specializes in chemical engineering process systems.
LUT’s calcium carbonate research recently claimed the first prize in the innovation competition FoRCe – Fortum Research Challenge. The research group is now in negotiations for industrial-scale application with Fortum and Soletair Power, headquartered on LUT’s Lappeenranta campus. The outcome may help to reduce production-related emissions and to increase material efficiency.
Atmospheric gases are found in satellites, phones and light bulbs
- Nitrogen: cold storage
- Oxygen: hospital oxygen tanks
- Argon: light bulbs, luxury car tires, double-glazed windows, 3D printing, welding
- Neon: neon advertising signs, television tubes, lasers, lighting systems in landscaping and interior design
- Helium: balloons, treatment of asthma, emphysema and other breathing problems, explosion prevention in industries
- Krypton: energy saving and fluorescent lights, flashes in high-speed photography, nuclear fusion energy research experiments, nuclear medicine for lung ventilation or perfusion scans, satellites
- Ammonia: fuel for transportation ships, cleaning solutions, component for heat transfer (the International Space Station uses ammonia to heat its exchangers)
- Xenon: antibacterial lamps in food preparation and processing, imaging of the heart, brain and lungs, ideal anesthetic gas
- Radon: cancer therapy and treatment of tumors, earthquake predictions, arthritis treatment
Sources: LUT University, European Environment Agency, www.vedantu.fi


Hydrogen from household waste
The best-known of the most recent applications for carbon dioxide is undoubtedly power-to-x (P2X) technology. Its outputs include plastics, hydrocarbons, and fuels such as methanol and methane. LUT is researching the manufacturing technologies of P2X chemicals to improve the energy and resource efficiency of processes and to reduce investment costs.
”We are conducting laboratory tests to collect data for process modeling and simulation. This will help us to design efficient production processes in collaboration with our partners,” Tuomas Koiranen continues.
In Koiranen’s view, the most interesting new initiatives in the utilization of carbon dioxide currently deal with carbon dioxide recovery from factory flue gases and the utilization of industrial hydrogen production by-products. Koiranen states that in the future, hydrogen could be manufactured from municipal waste and by-products from wood refining and the metal industry.
“Flue gas purification and the production of clean carbon dioxide are highly important to industry because the production of synthetic fuels, chemicals and plastics requires the cost-efficient removal of impurities such as nitrogen and sulphur,” says Koiranen.
The economically and ecologically sustainable production of carbon dioxide for industrial use poses researchers a challenge that demands novel, process-efficient solutions. LUT’s new gas research laboratory opens up new opportunities also in this strain of research.
Fertilizers from ammonia to secure food chains during conflicts
Ammonia is widely used in the fertilizer industry. It’s conventionally produced in the oil and gas industries using high pressure and temperature reactions that demand a high energy input. However, ammonia could also be produced from air and water under more moderate conditions. This is something Assistant Professor Nima Rezaei wants to research.
“About 2% of the global energy consumption is used for the production of ammonia. This also produces 1% of the world’s CO2 emissions. Therefore, there’s a need to develop a more sustainable and green process,” Rezaei concludes.
Urea is a nitrogen-based fertilizer that accounts for 80% of global ammonia use. Russia’s war in Ukraine has increased interest in sustainable and local fertilizers due to related disruptions in energy and material supply chains. Given that Ukraine and Russia dominate the global fertilizer market, any disruptions in fertilizer supply chains will threaten food security. Therefore, producing ammonia locally for energy use and fertilizers with sustainable resources will have strategic value.


Xenon – the anesthetic gas of the future
Xenon is a rare atmospheric gas which is used in photographic flashes of smartphones and cameras and in satellite propulsion systems that redirect the satellite into the desired orbit. The atmosphere is a very important source of xenon because there is currently no practical alternative. Xenon is distilled from air by lowering the air temperature to induce liquification. Then, the different components are distilled based on their boiling points.
“The atmosphere contains 5000 times more CO2 and 10 million times more nitrogen than xenon. Air distillation to produce xenon is a very expensive and energy intensive process because its concentration in the air is very low,” Rezaei explains.
According to Rezaei, xenon is an ideal anesthetic gas that is especially well suited for more vulnerable patients such as infants, elderly people, and people with cardiovascular diseases. However, because the xenon content in the atmosphere is low and air distillation is difficult, it is critically important for recycling to separate xenon efficiently from contaminants such as carbon dioxide and water vapor.
“It will be an interesting new research topic, because xenon is and will be very difficult to obtain. Its recycling and purification will significantly increase sustainability and decrease its carbon footprint.”
At present, the most common gases in anesthesia are nitrous oxide and highly fluorinated gases, which have negative impacts on the health of both patients and medical personnel. In addition, nitrous oxide has a high global warming potential – nearly 300 times that of carbon dioxide – whereas fluorinated gases deplete ozone.
In a nutshell: How were all the gases formed in the atmosphere?
The Earth’s atmosphere was first formed 4.5 billion years ago. However, the current atmosphere is only about 400 million years old. Different gases have formed during this evolution, which, to simplify, took four steps:
- First, the atmosphere consisted only of hot hydrogen and helium. Without the Earth's gravity, these gases would have escaped.
- Erupting volcanoes spewed carbon dioxide, water vapor, ammonia, methane and sulfur dioxide into the air. The reaction between ammonia and trace oxygen at a high temperature produced nitrogen. Methane reacted with trace oxygen and produced carbon dioxide.
- About 3.8 billion years ago, the Earth’s surface started to cool down in two stages: A hot planet collided with the Earth. The Earth’s moon was formed out of the particles that scattered. Then the temperature of the Earth's surface dropped below 100 degrees, which allowed water vapor to condense. This created water bodies such as oceans and rivers into which carbon dioxide and other gases dissolved.
- Finally, bacteria started to thrive 3 million years ago. Over time, these species became more and more complex and started to release oxygen. The oxygen reacted with UV rays, forming the ozone layer. That, in turn, increased the activity of plants, bacteria and algae and shaped the atmosphere that now surrounds us.
Source: Assistant Professor Nima Rezaei
More information

Nima Rezaei






